Background:
Even in the BCMA CAR-T era, multiple myeloma (MM) patients with high-risk genotypes have the poorest outcomes. We thus leveraged MMRF CoMMpass data to identify potential novel surface immunotherapeutic targets in high-risk MM. As described below, we focused on CD70, an immunotherapeutic target known to be upregulated on many hematologic and solid tumors but minimally expressed on normal tissue. While CD70 was previously explored in MM (McEarchern et al, Clin Cancer Res (2008)), it was not further investigated due to heterogeneous expression in newly diagnosed samples. Here we revisit this target to suggest cellular therapies against CD70 could be highly beneficial in high-risk MM patients, currently the greatest unmet need in the field.
Methods:
Primary patient samples were obtained under an IRB-approved protocol. CAR-T lentiviral transduction was performed on paired CD4/CD8 human T cells from at least 3 healthy donors. Effector function was assessed by cytotoxicity against MM cell lines and CAR-T profiling for memory and exhaustion markers. In vivo efficiency was assessed using bioluminescence. ATAC-seq peaks were called using Promo and HOMER (data from Jin et al. Blood (2018)). Machine learning was performed using XGBoost approach applied to CoMMpass RNA-seq data with 80% of samples for training and 20% as test set.
Results:
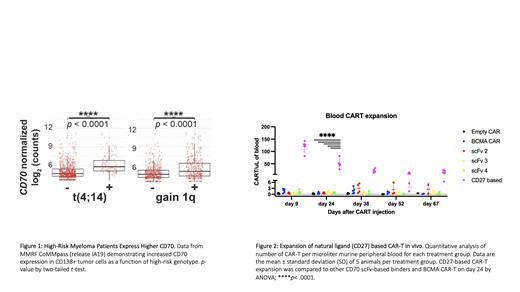
Analyzing CoMMpass mRNA data (release IA19) we first evaluated surface protein encoding-genes upregulated in tumors at relapse compared to diagnosis. Among >100 significantly upregulated genes, we focused on CD70 given known promise as an immunotherapy target. Further analysis across genomic subtypes revealed a specific, marked upregulation of CD70 in t(4;14) and gain 1q ( Fig. 1), as well as R-ISS 3 at diagnosis.
Flow cytometry profiling of 8 cell line models, including t(4;14) models KHM11, KMS26, and LP1, revealed consistent CD70 protein expression with antigen density similar to BCMA. Profiling of relapsed primary MM samples confirmed that 19 of 28 samples had CD138+ plasma cells positive for CD70 expression. Of 5 patients in whom both BCMA and CD70 were measured, antigen densities were also similar.
To target CD70, we then designed 10 unique anti-CD70 CAR-T constructs. 9 of these were scFv-based binders derived from therapeutic antibody fragments currently in preclinical or clinical development. By a structure-guided design based on the CD70:CD27 co-crystal structure (PDB: 7KX0), we also derived a unique truncated fragment of CD27 as an alternative CD70 binding element. All CD70 CARs were efficacious in in vitro cytotoxicity assays targeting MM cell lines AMO1, MM1.S, LP1, and RPMI8226. We then selected the top 3 performing scFv based-CARs as well as our natural ligand (CD27) CAR-T for in vivo studies. All 4 CD70 CAR-Ts prolonged survival of MM.1S-bearing mice compared to empty CAR ( n=5 mice/arm, p<0.005 by log-rank), similar to BCMA CAR-T controls. Remarkably, the CD27-based CAR showed superior expansion (50-85x, p< 0.0001 by ANOVA) and persistence compared to scFv-based ( Fig. 2). These results underscore that targeting CD70 using a natural ligand-based CAR-T represents a promising preclinical candidate for treatment of relapsed and high risk MM.
We further investigated why CD70 may be upregulated in high risk MM. Analysis of ChIP-seq in ENCODE and primary MM tumor ATAC-seq was used to nominate a set of transcription factors that bind the CD70 locus. Extending from CoMMpass data, we built a predictive machine learning model for CD70 expression as a function of transcription factor gene expression, nominating key transcription factors TFAP2A, LEF1, MYB, and PAX5 as CD70 regulators; validation is now underway. Additionally, we showed that knockout of NSD2 in t(4;14) models KMS34 and KMS11 led to significantly decreased CD70 surface expression, implicating this central epigenetic driver in CD70 overexpression in this high-risk MM subtype. Finally, we also demonstrated that treatment with the hypomethylating agent azacytidine can drive increased CD70 expression, similar to that shown in AML models.
Conclusion:
Taken together, targeting CD70 appears to be a highly promising cellular therapeutic strategy for MM, with particularly high utility in high-risk patients after BCMA CAR-T relapse. We are continuing preclinical and future clinical development of this CAR-T strategy, using our natural ligand CD27 design with extended in vivo persistence.
Disclosures
Boise:Abbvie: Consultancy; Astra Zeneca: Consultancy, Honoraria. Licht:AstraZeneca: Consultancy; Epizyme: Research Funding. Wiita:Genentech/Roche: Research Funding; Sanofi: Honoraria.